David Chalmers, writing in “Three Puzzles About Spatial Experience,” calls extrinsicalism the “consensus” position, while David Baker, writing in “‘The Experience of Left and Right’ Meets the Physics of Left and Right,” says “I speak for a large population of specialists in asserting that [extrinsicalism] is far more plausible than [intrinsicalism].” In his honors thesis, however, Eli synthesized research from physical biochemistry and the Standard Model of particle physics to argue in favor of the contrary intrinsicalist thesis: that incongruent counterparts differ with regard to some non-relational intrinsic property.
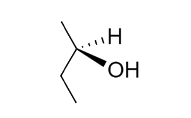
In organic chemistry, a compound is said to be chiral when it contains one or more chiral centers, carbon atoms that are bonded to four or more different chemical groups. Take, for example, the compound R-2-butanol:
To the chemist, chiral compounds are notable for the fact that they are always members of incongruent pairs; together, these non-superimposable, mirrored molecules are called enantiomers. The “R” in R-2-Butanol, in fact, denotes a specific enantiomer of 2-Butanol, with its corresponding mirror image denoted with an “S.”
For much of chemical history, enantiomers were believed to differ only in relation to one another. In the mid-1950s, however, the work of physicist Chien-Shiung Wu began to call this orthodoxy into question: through a ground-breaking series of experiments, Wu and her colleagues demonstrated that electroweak forces treat incongruent counterparts differently. Soon after the publication of Wu’s results, theoretical biologists began to predict that these electroweak forces would cause enantiomers—such as R-2- and S-2-butanol—to differ, particularly in the metric of Gibbs Free Energy (G). G is a thermodynamic quantity, describing the amount of reversible work that may be done by a given isobaric and isothermal system; in the context of biochemistry, G can roughly be thought of as the amount of energy stored in a molecule’s bonds. Over the fifty years since this initial proposal, an increasingly complex series of studies has attempted to quantify the difference in Gibbs Free Energy between enantiomers (referred to as ΔG), with specific focus on the differences between carbohydrate and amino acid enantiomers. These studies first approached the question of quantifying ΔG from a theoretical perspective, employing ab initio calculations that take only physical constants as inputs and make no assumptions about a compound’s environment. By these calculations, ΔG is incredibly small—10-12 J/mol to 10-15 J/mol—but a ΔG due to electroweak forces is nonetheless universally regarded as extant. While the direction of the inequality is a matter of scientific debate, the existence of a difference, under any set of conditions, is not.
If the phenomenon of ΔG is to constitute an objection to the extrinsicalist thesis, however, G must be an intrinsic property that can be defined without reference to an external object. At a fundamental level, this can be accomplished through two different forms of argument. First, we can argue for this claim from an empirical standpoint. For a given compound under any set of constant conditions, G itself is a constant and characteristic quantity. Since G is essentially a measure of the energy contained in the bonds of a compound, any two compounds with the same G will have the same bonds in the same orientations. If G is defined in terms of a compound’s bonds, no object external to the compound is required to define G, and G, therefore, is an intrinsic property. However, we can also look at this question from a more intuitive perspective and echo back to a classic example considered by Kant: if the universe contained only one enantiomer of a molecule, could we determine whether it was the R- or S-enantiomer? The answer arising from research on ΔG leads us to answer affirmatively. ΔG entails a difference between the G of enantiomers, and as such, a precise enough analysis of our lone enantiomer’s energy could be used to determine its R- or S-enantiomerism. Again, we are guided to the conclusion that G is intrinsic, as no other object in the universe is needed to define G, or further, to distinguish one enantiomer from the other. By considering both of these arguments, we have grounds to believe that G is in fact intrinsic, and insofar as research on ΔG entails a difference in G between enantiomers, we have also motivated the intrinsicalist thesis.
Though this finding directly relates to the philosophy of physics, the conclusion Eli developed has implications in phenomenology and epistemology. In the context of phenomenology, the extrinsicalist thesis has been applied by numerous authors to advance or analyze surprising conclusions about the structure of left/right experiences. In “A puzzle about the experience of left and right,” for example, Brian Cutter develops a paradox between the phenomenology and metaphysics of orientation through reference to extrinsicalism, arguing for a disjunctive conclusion: either there is a degree of independence between phenomenology and what our experience represents, or spatial properties presented in perception are mind-dependent or illusory. But if extrinsicalism is abandoned in favor of the intrinsicalist thesis, this paradox vanishes. In the context of epistemology, David Chalmers, writing in “Three Puzzles About Spatial Experience,” seeks to motivate the impossibility of lifelong illusions about orientation through an appeal to the extrinsicalist thesis; but if the extrinsicalist thesis fails, Chalmers cannot reach his conclusion. These considerations, when taken together, have significant impacts in the philosophy of physics, in how we understand perception, and in epistemology, supporting skeptical hypotheses in each of these fields.
After completing his undergraduate degree in Philosophy at Indiana University, Eli G. Schantz is currently a medical student at Indiana University School of Medicine—South Bend, where his research centers on ontology and its application to debates in bioethics and the philosophy of medicine. He also serves as a delegate to the Indiana State Medical Association and the Medical Student Section of the American Medical Association, where he sits on the AMA-MSS Committee on Bioethics and Humanities and advocates for ethics-informed healthcare policy.


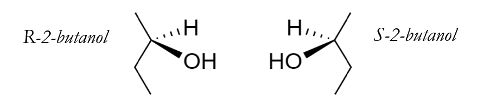
 The College of Arts
The College of Arts